You can now add Healthify as a preferred source on Google. Click here to see us when you search Google.
Duolin
Also called ipratropium + salbutamol
Key points about Duolin
- Duolin® is used to treat conditions where breathing is a problem, such as COPD.
- Ipratropium + salbutamol sounds like 'ip-ra-TRO-pee-um' and 'sal-BYOO-ta-mol'.
- Find out how to take it safely and possible side effects.

Duolin® is a combination of two medicines, ipratropium and salbutamol, mixed together in a puffer or inhaler. The combination is used to treat conditions where breathing is a problem, such as COPD, chronic bronchitis and emphysema. It works by relaxing and opening up the air passages, making breathing easier and improving shortness of breath, chest tightness and wheezing.
Duolin belongs to a group of medicines known as bronchodilators or relievers (it's called a reliever medicine because it quickly relieves your breathing problems).
Using an inhaler device enables the medicine to go straight into your airways when you breathe in. This means that your airways and lungs are treated, but very little of the medicine gets into the rest of your body.
In Aotearoa New Zealand, Duolin is available as an inhaler and nebulising solution. Nebulisers are used when using an inhaler isn't suitable. The information on this page is about Duolin inhaler. Read more about nebulisers.
Brand change: Nebulising solution
From 1 February 2026, the funded brand of Duolin nebulising solution will temporarily change due to supply issues. The new funded brand is Cipla.
Cipla nebulisers have the same active ingredients, ipratropium and salbutamol, in the same amounts as Duolin and should work in the same way.
- Different packaging: The packaging of Cipla is different. It comes as vials (instead of ampoules) and each vial is 3 mL (instead of 2.5 mL).
- Different name: The vials inside the foil pack are labelled “albuterol”. Albuterol is the US name for salbutamol. The vials are labelled “albuterol 3 mg” – this is the same dose as 2.5 mg salbutamol.
- Cipla is not approved by Medsafe, but it is approved in the United States. Read more about unapproved medicines.
- The stock of Cipla nebulising solution expires in July 2026, so it is intended as a short-term alternative while the Duolin supply issue is resolved.
For more information about this change, including images of the Cipla box label, visit Pharmac: Duolin nebuliser supply issue(external link).
If you have any questions about this brand change, talk to your healthcare provider.
Note: This brand change does not affect the Duolin inhaler.
- Follow your doctor's instructions carefully. The pharmacy label on your medicine will tell you how much to use, how often to use it and any special instructions.
- The usual dose of the Duolin inhaler is 2 puffs 4 times a day. You should not use more than 12 puffs in any 24 hour period.
Use your MDI with a spacer
- A spacer is an attachment to use with your inhaler. It's a chamber that holds the medication before you inhale, giving you a more controlled, deeper inhalation. This can be especially helpful if you're short of breath.
- Using your MDI with a spacer makes it easier to use the inhaler and helps to get the medicine into your lungs, where it’s needed (with less medicine ending up in your mouth and throat).
- Read more about spacers.
Check your technique
- To get the most benefit from your inhaler, it's important to use the correct technique.
- Ask your healthcare provider to show you how to use your MDI and spacer devices.
- Even if you’ve been shown before, ask your healthcare provider to explain how to use your inhaler if you still have any questions.
The video below provides some guidance on how to use a spacer with your MDI. Note: Your MDI or spacer may look different to the one in the video below.
Video: How to use your spacer device
(Healthify He Puna Waiora, NZ and Auckland District Health Board, 2018)
Like all medicines, Duolin can cause side effects, although not everyone gets them. Often side effects improve as your body adjusts to the new medicine.
| Side effects | What should I do? |
|---|---|
|
|
|
|
|
|
|
|
|
|
Read more about medicines and side effects and reporting a reaction you think might be a side effect.
Salbutamol and ipratropium(external link) New Zealand Formulary Patient Information
Regional Blue card COPD Action plan [PDF, 355 KB] District Health Boards, NZ, 2019
5 questions to ask about your medications(external link) Health Quality and Safety Commission, NZ, 2019 English(external link), te reo Māori(external link)
References
- Ipratropium bromide + salbutamol(external link) New Zealand Formulary
Brochures

My Medicines, NZ, 2017
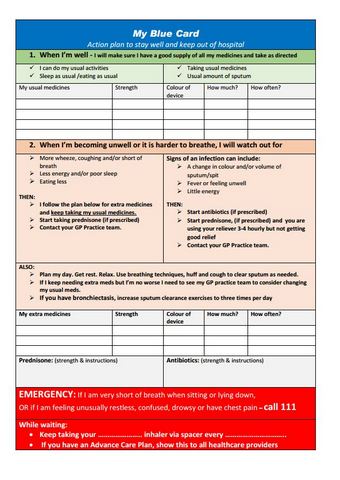
Regional Blue card COPD Action plan
District Health Boards, NZ, 2019

Medicines and side effects
Healthify He Puna Waiora, NZ, 2024
Credits: Sandra Ponen, Pharmacist, Healthify He Puna Waiora. Healthify is brought to you by Health Navigator Charitable Trust.
Reviewed by: Angela Lambie, Pharmacist, Auckland
Last reviewed:
Page last updated:





