You can now add Healthify as a preferred source on Google. Click here to see us when you search Google.
Generic medicines
Key points about generic medicines
- A generic medicine contains the same active ingredient, in the same quantity, as the original brand.
- They work in the same way as brand medicines but are cheaper and may look different.
- Generic medicines have to have the same active ingredient in them as the original medicine, they have the same risks and benefits as the original brand medicines.

- When a pharmaceutical company first develops a medicine, it takes out a patent to make sure it has the exclusive right to produce and market the medicine with a particular active ingredient.
- When the patent for the medicine expires, other manufacturers can also make the medicine with the same active ingredient. These are generic medicines.
- All generic medicines have to be approved by Medsafe and go through bioequivalence testing to make sure they work the same way as the original brand of that medicine.
- Because generic medicines have to have the same active ingredient in them as the original medicine, they have the same risks and benefits as the original brand medicines.
- Generic medicines can look different because their inactive ingredients may be different to the inactive ingredients in the original medicine
- While a generic medicine costs less, it will still work as well as the more expensive brand-name medicine.
- Generic medicines are often produced by major pharmaceutical companies that also develop original brand name medicines.
When a pharmaceutical company first develops a medicine, it takes out a patent to make sure it has the exclusive right to produce and market the medicine with a particular active ingredient. The active ingredient is the chemical in the medicine that makes the medicine work.
These original medicines are often marketed under the pharmaceutical company’s trademarked name or brand name and can be called the ‘originator brand’ or the ‘branded’ medicine.
When the patent for the medicine expires, other manufacturers can also make the medicine with the same active ingredient. These are generic medicines.
Some branded products are made using a patented production process, rather than a patented active ingredient. In such cases, generic products can be made but must use different production processes that do not infringe the patent.
Yes. Generic medicines have to be ‘bioequivalent’. This means that they have to have the same active ingredient, and that ingredient has to be delivered to the body in the same way and have the same effect on the body as the original medicine. They don’t have to be manufactured using the same process. 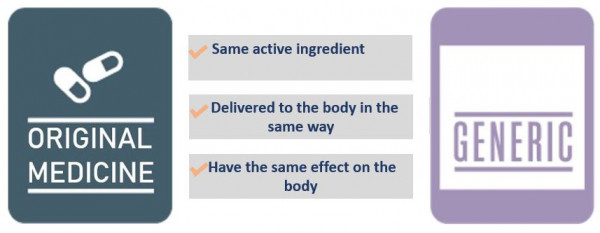
Generic medicines may be different from the brand name version in:
- shape, size and colour
- packaging
- 'inactive ingredients' that do not contribute to the treatment effect of the medicine.
Yes. Medsafe, a unit of Health New Zealand | Te Whatu Ora that regulates medicines used in New Zealand, makes sure that all medicines available in New Zealand go through the same quality, efficacy and safety checks before they can become available. Because generic medicines have to have the same active ingredient in them as the original medicine, they have the same risks and benefits as the original brand medicines.
In most cases PHARMAC funds generic medicines that Medsafe has assessed as being safe, effective and of good quality. PHARMAC gets advice from clinical advisory committees before it considers tendering for a generic medicine.
For more information about the tender process, see Medicine and medical devices contract negotiation(external link)
In all medicines there are both active and inactive ingredients. The inactive ingredients in the medicine are called excipients. These inactive ingredients hold the medicine together, give the medicine its colour or make it easier to swallow. Generic medicines can look different because their inactive ingredients may be different to the inactive ingredients in the original medicine.
Sometimes people can be allergic to excipients, both in brand name and generic medicines. If you are allergic to something it is important to check what your medicine contains, regardless of whether you are taking a branded or a generic medicine.
It often costs pharmaceutical companies a lot of money to develop and market a new medicine, and these costs are then passed on to the people who buy the medicines. The company that makes the original medicine takes out a patent so they are the only ones who can make this medicine, or make it using a particular process. This helps the company recover some of the costs associated with developing and marketing their new medicine.
Companies making generics do not have to make the medicine from scratch or pay for the research (in most cases), develop the medicine or carry out the same range of clinical trials that the original medicine has to go through. That means it costs less for them to put the medicine on the market.
Generic medicines are also cheaper because of competition. When different makers of a medicine are competing against each other prices come down.
No. Because they have the same active ingredient and are delivered to the body in the same manner, generic medicines work in the same way and in the same amount of time as the original brand medicine.
Generic medicines are produced in factories all over the world. These factories must be approved by international regulators, including Medsafe, before they can provide medicines to New Zealand. Often, the factories that produce generic medicines are also producing the active ingredient for brand name medicines.
It doesn’t matter where in the world the factory producing the generic medicine is, the approval process and the standards for the medicines are the same.
Generic medicines are often produced by major pharmaceutical companies that also develop original brand name medicines.
Many brand name medicine companies are now buying or going into partnership with generic medicines companies to expand the range of products they produce and broaden their revenue base.
Brochures

Medicines and side effects
Healthify He Puna Waiora, NZ, 2024

Health Quality and Safety Commission, NZ, 2019 English, te reo Māori
Credits: Healthify He Puna Waiora editorial team. Healthify is brought to you by Health Navigator Charitable Trust.
Page last updated:





