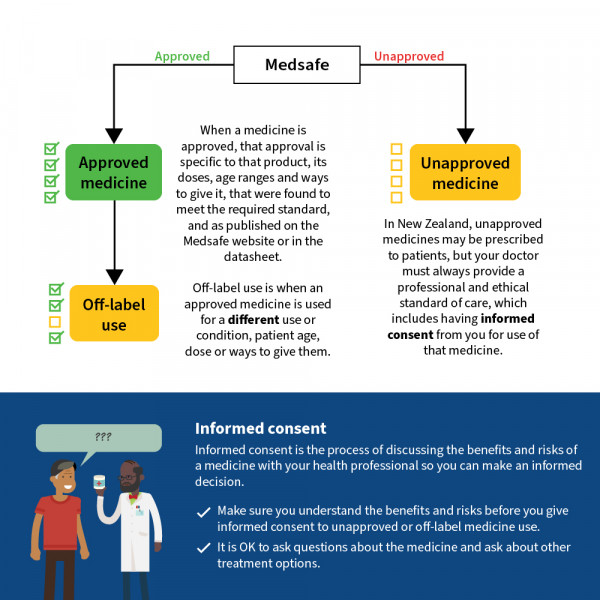
In Aotearoa New Zealand most medicines you are prescribed are approved medicines. This means they have been through a regulatory assessment process in New Zealand to ensure that the quality of the product meets the required standard and that the product can be considered safe and effective for the uses listed in that medicine’s data sheet.
Data sheet
A data sheet is a document that is written by the drug company for healthcare professionals and has information about the medicine, including the details for which the medicine is approved for use in New Zealand, such as the specific uses, doses, age ranges and routes (ways that a medicine is given).
Most companies also provide consumer medicine information (CMI) for patients. Data sheets and CMI for medicines are available on the Medsafe website(external link). Medsafe is the New Zealand Medicines and Medical Devices Safety Authority, and is a part of Health New Zealand | Te Whatu Ora. It is responsible for the regulation of medicines.
Before a medicine can be approved
Before a medicine can be approved, the drug company must submit clinical and scientific data to Medsafe for review. The drug company must show that the medicine is of acceptable quality and is safe and effective for its intended uses.
‘Safe’ does not mean that the medicine has no side effects. Instead, it means Medsafe has determined that the benefits of using the medicine for a particular use outweigh the potential risks overall. For some individual patients this may not be true, which is why your doctor, nurse or pharmacist considers carefully which medicines are best for you.
When a medicine is approved
When a medicine is approved, that approval is specific to the uses, doses, age ranges and routes (ways that a medicine is given) that were found to meet the required standard, and as published on the Medsafe website or in the data sheet.
If there are any changes, such as a new use or dose, this must be applied for by the drug company and approved by Medsafe.